The discovery of autoantibodies against the phospholipase A2 receptor 1 (PLA2R) is considered a milestone in nephrology research. They are the first described serological marker for primary membranous nephropathy. Their value in membranous nephropathy diagnostics is the subject of many recent publications.
Primary and secondary membranous nephropathy: differential diagnosis essential
Membranous nephropathy is the major cause of nephrotic syndrome in adults, which is an inflammatory condition of the glomeruli. Its characteristic symptoms are an increased excretion of proteins in the primary urine (proteinuria) as well as dysfunctioning of lipid metabolism (hyperlipidemia, lipiduria). The disease can take different courses of severity ranging from spontaneous remission or persistent proteinuria to kidney failure.
In 20% – 30% of membranous nephropathy patients, the inflammation is caused by another underlying disease, e.g. tumour, autoimmune diseases, bacterial or viral infections or drug intoxication. This form is called secondary membranous nephropathy. Primary membranous nephropathy, in contrast, is an independent, organ-specific autoimmune disease. Differentiation of the two membranous nephropathy types during diagnosis is crucial for an appropriate therapy. While treatment of patients suffering from secondary membranous nephropathy is directed to the underlying disease, patients presenting with primary membranous nephropathy are usually treated with immunosuppressive therapy.
To date, diagnosis of membranous nephropathy is based on results from kidney biopsy. If positive, the histological examination of the kidney tissue reveals immune complex deposits on the outer side of the glomerular basement membrane. Patients with secondary membranous nephropathy exhibit antibodies within the immune deposits which are indicative for the underlying disease, e.g. antibodies against double-stranded DNA in Lupus erythematodes or against Treponema – and Helicobacter pylori antigenes in the corresponding infectious diseases. The target antigen being involved in the formation of immune deposits causing primary membranous nephropathy, however, have been discovered only recently. In 2009, Beck (et al.) reported on the presence of autoantibodies targeting the phospholipase A2 receptor 1 (PLA2R) specifically in patients with primary membranous nephropathy. Approximately 70% of the examined primary membranous nephropathy patients revealed anti-PLA2R autoantibodies in their sera [1].
Anti-PLA2R and pathogenesis of primary membranous nephropathy
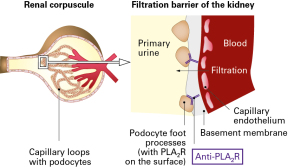
PLA2R is a membrane spanning receptor with a large extracellular domain, which is expressed in podocytes of the glomeruli. According to the literature, the anti-PLA2R antibodies may play an important role in the pathogenesis of primary membranous nephropathy. Likely, the circulating anti-PLA2R antibodies bind to the extracellular domain of the receptor on the podocyte surface. This may lead to local complement activation and inflammation and destruction of the glomerular basement membrane, resulting in disruption of kidney filtration [2].
The value of anti-PLA2R in clinical practice
Recent studies have confirmed the specific appearance of anti-PLA2R antibodies in primary membranous nephropathy patients and therewith their function as a biomarker for primary membranous nephropathy. Patients suffering from secondary membranous nephropathy do not exhibit anti-PLA2R autoantibodies by the majority [3, 4]. In the few described cases of anti-PLA2R positive secondary membranous nephropathy, a parallel incidence of a (primary) membranous nephropathy and a second independent disease could not be excluded [2].
Additionally, the anti-PLA2R antibody titer is also suitable as a prognostic marker to evaluate and monitor the disease course and therapy. There is a significant correlation between the anti-PLA2R antibody titer and the disease activity. High titers are associated with a more severe course of the primary membranous nephropathy which requires a more intensive therapy. When patients are treated with immunosuppressive drugs, the titer rapidly decreases – faster than the proteinuria. The chance of therapeutic success and remission of primary membranous nephropathy is higher if the initial anti-PLA2R antibody titer had been low [5].
Furthermore, a low anti-PLA2R titer seems to increase the probability of remission after kidney transplantation. A persistent presence of anti-PLA2R autoantibodies after organ transplantation has been identified as significant risk factor for primary membranous nephropathy relapse in a recent study [6].
Anti-PLA2R autoantibodies, therefore, possess a high diagnostic value for primary membranous nephropathy. Determining the anti-PLA2R antibody titer further allows evaluation and prediction of membranous nephropathy activity, necessary therapeutic measures and chances of therapy success.
Testing anti-PLA2R autoantibodies
There are only two standardized test systems for the qualitative and quantitative detection of anti-PLA2R autoantibodies which are available in Europe and the US: Anti-PLA2R IIFT and Anti-PLA2R ELISA [7, 8]. In contrast to biopsy, the application of these serological tests does not require invasive surgery, but merely a small blood sample.
Further information about anti-PLA2R autoantibodies and their role in primary membranous nephropathy as well as about publications and events on this subject are provided on the PLA2R website.
[1] Beck et al., 2009 N Engl J Med, [2] Ronco and Debiec, 2012 Nat Rev Nephrol, [3] Hofstra et al., 2011 Clin J Am Soc Nephrol, [4] Gunnarsson et al., 2012 Am J Kidney Dis, [5] Hofstra et al., 2012 J Am Soc Nephrol, [6] Seitz-Polski et al., 2014 Nephrol Dial Transplant, [7] Hoxha et al., 2011 Nephrol Dial Transplant, [8] Dähnrich et al., 2013 Clin Chim Acta