Since the last large Zika fever epidemic in Brazil 2015/2016, during which an association of the infectious disease with severe neurological syndromes was identified (Guillain-Barré syndrome in adults, congenital neurological damages in newborns), the demand for reliable diagnostic tests has increased extremely. With the new PCR-based direct detection, EUROIMMUN complements the existing range of serological test systems in order to enable reliable laboratory diagnostics in every stage of infection.
PCR-based direct detection
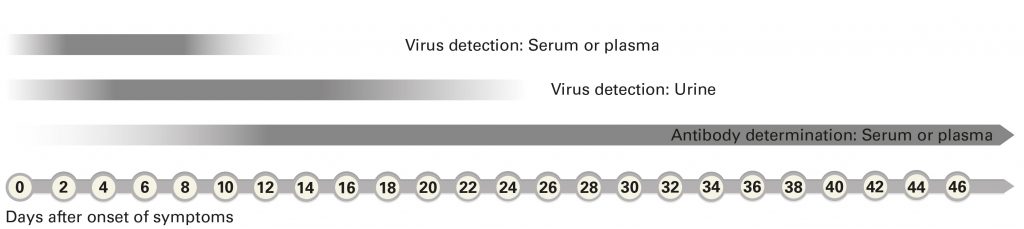
PCR-based test systems can detect virus RNA in a patient sample withdrawn during the acute (viraemic) phase of the infection. Zika virus RNA can be reliably detected in serum from day 1 to 5, in urine up to day 10 after onset of first symptoms. In individual cases, this time span can be up to 2 (serum) or 3 (urine) weeks. However, specific antibodies which can be determined by serological methods are produced from around day 7 and are then detectable for several months or years in the patient’s serum.
PCR-based direct detection is therefore only relevant for diagnosis at the early stage of infection. Since it is based on the detection of the genome sequences which are unique of the virus, it is also highly specific: cross-reactions with genome sequences of other arboviruses which cause similar symptoms and are endemic in the same regions, such as dengue or chikungunya virus, can be excluded.
EURORealTime Zika virus
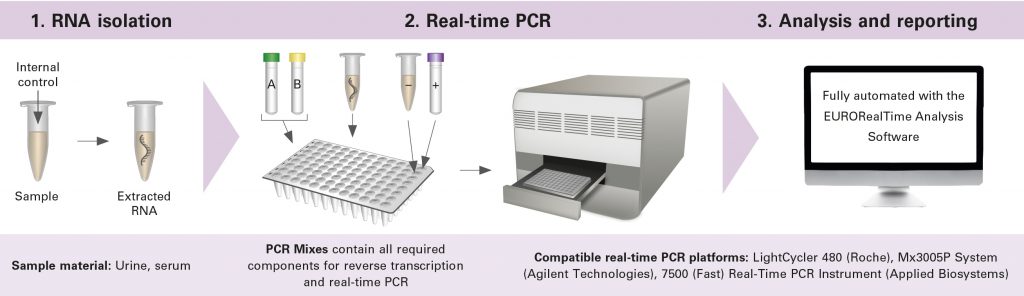
The new EURORealTime Zika Virus test combines reverse transcription of the Zika virus RNA into (complementary) DNA and the subsequent real time PCR in one assay. In the real time PCR, the selected sequence parts of the Zika virus genome are exponentially multiplied and the PCR products labeled with a fluorescence dye, so that the amplification process can be followed in real time.
The entire test procedure is accompanied by the EURORealTime Analysis software which guides through all the required work steps, thus preventing errors. The subsequent evaluation is also done by the software: after the automated and standardised analysis of the raw data, it generates a result report and documents and archives the results. The EURORealTime Analysis Software is compatible with many different real time PCR platforms and laboratory information systems.
| n = 55 | CE-labeled Zika virus real time PCR test | ||
| positive | negative | ||
| EURORealTime Zika Virus | positive | 20 | 1 |
| negative | 1 | 33 | |
An external comparative study of the EURORealTime Zika Virus test and another CE-labeled Zika virus PCR test was performed in Brazil using 29 serum and 26 urine samples from patients with suspected Zika virus infections. It yielded an agreement of the positive and negative results of 95.2% and 97.0%, respectively.
The EURORealTime Zika Virus test is the first product of EUROIMMUN‘s new product line for direct detection, EURORealTime ̶ further tests for the diagnosis of infectious diseases will follow soon.