EUROIMMUN has been offering test systems for therapeutic drug monitoring (TDM) since 2019. Owing to the cooperation with IDS and their range of TDM products from Theradiag, we were able to significantly expand our portfolio: We are now offering ELISA and ChLIA test systems for the therapeutic monitoring of different biologics and antibodies against them (so called anti-drug antibodies). This enables the individualisation of treatment with biologics and contributes to a prompt therapy success.
Treatment with biologics and therapeutic drug monitoring
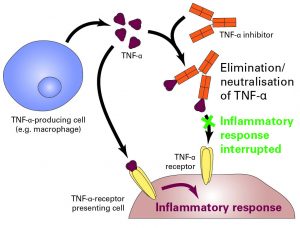
Biologics are applied to treat a number of inflammatory autoimmune diseases, such as chronic-inflammatory bowel diseases, rheumatic diseases, psoriasis and multiple sclerosis, in which different molecular mediators are involved in the inflammatory processes. Biologics are similar to the body’s own substances and can therefore influence the regulatory processes of the body in a targeted manner. For instance, there is the large group of TNF-α inhibitors, which can interrupt the endogenous inflammatory cascade on the level of the underlying messenger substance TNF-α: The inhibitors, monoclonal antibodies, inhibit the binding of TNF-α to its specific receptor by binding to it and thus stopping the inflammatory response (see figure).
Since biologics are very expensive preparations, some of them even requiring intravenous administration, they are only administered to those patients who do not respond sufficiently to conventional medication or who cannot be treated by conventional medication due to contraindications.
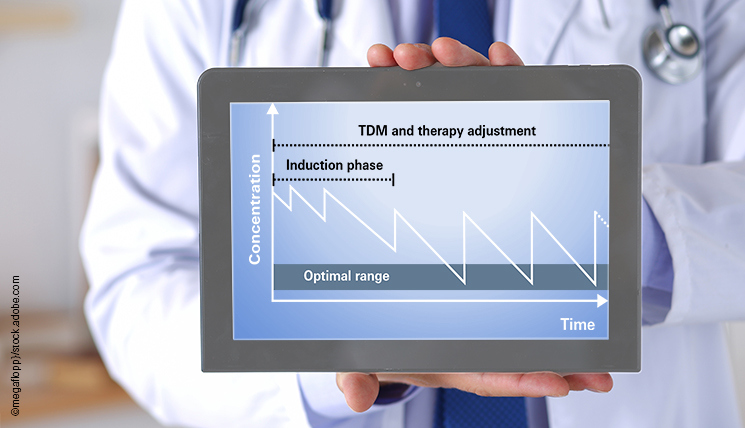
If a patient is to be treated by biologics, the drug dosage must be individually adapted in order to achieve the most effective concentration of the drug in the blood. Optimal treatment can only be achieved if the drug level is within the therapeutic window. The concentration of the active agent depends on different factors such as size, age, sex, pre-existing conditions, etc., and may therefore vary greatly from person to person despite the identical drug dose. This is referred to as the pharmacokinetics of the active agent, i.e., its individual uptake and processing in the body. In the optimal range, the lowest possible drug level in the blood achieves maximum improvement of symptoms. The concentration of the active agents is monitored in the so-called therapeutic drug monitoring (TDM).
Strategies of therapeutic drug monitoring (TDM)
TDM can be performed based on two different strategies:
With proactive TDM, the drug level is regularly monitored in patients who respond well to the treatment (responders). In this way, potentially too high, so-called supratherapeutic drug levels can be recognised early. At that level, an increase in the drug dose no longer leads to an improvement of the symptoms. In such cases, the individual doses can be reduced, or the administration intervals increased, especially in order to reduce the risk of potential side effects and to save time and costs.
With reactive TDM, the drug levels are monitored in patients who do not respond to the therapy (non-responders). Here, the aim is to find out why the therapy is not working. For example, the drug concentration may be below the optimal range despite the recommended dose being administered, due to the patient’s individual pharmacokinetics. In such cases, the single doses can be increased or the time interval between administrations reduced. If the concentration is below a critical threshold value, the presence of anti-drug antibodies should be investigated.
Failing treatment due to anti-drug antibodies
Almost all patients under immunointerfering therapy present anti-drug antibodies (ADA) at an earlier or later stage of treatment. These bind the active agent so that it is eliminated prematurely from the body or its effectiveness is reduced. Especially in high concentrations, ADA may cause a drug to fail, so that a change of the drug or active agent is required. The concentration of free ADA (i.e., that are not bound to an active agent) can be determined quantitatively with the corresponding test systems.
Take-home message
There is no panacea for the treatment with biologics. Many factors influence the effectiveness of the drugs, which is why the treatment must be individually customised for every patient. Here, TDM can be a great support: It can help to ensure the best outcome of treatment with biologics.
Test systems for therapeutic drug monitoring
The ELISA and ChLIA product portfolio allows both the specific determination of concentrations of different biologics (e.g. Adalimumab, Infliximab, Ustekinumab, Vedolizumab, Golimumab, Rituximab, Certolizumab Pegol) and their biosimilars (similar drugs with the same mode of action as the original preparation) as well as detection of the respective ADA. Blood serum and plasma are used as sample materials.
Please find further information on the products here.