On 15th April 2025, the European Commission authorised lecanemab (monoclonal anti-amyloid (beta) antibody; trade name: Leqembi from Eisai and Biogen) in the European Union to treat early Alzheimer’s disease.
A decision was long awaited after the positive opinion on the marketing authorisation of the medicine by the European Medicines Agency (EMA) in November 2024.
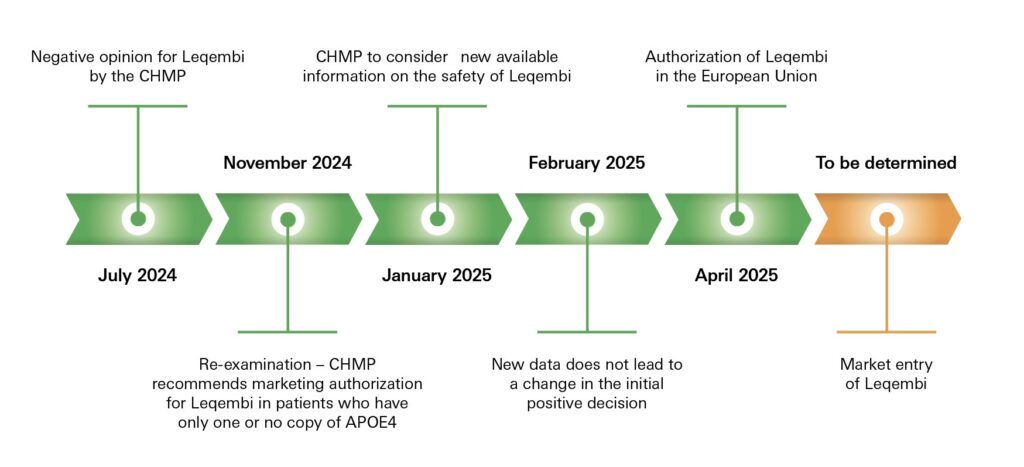
The European Commission now follows the recommendation of the EMA, likewise restricting the use of lecanemab for treating only Alzheimer’s patients in early disease stages (mild cognitive impairment) and with just one or no copy of the ɛ4 allele of the apolipoprotein E (APOE) gene.
This is because clinical studies revealed that patients carrying two copies of APOE ɛ4 show the highest risk among all APOE genotypes for developing potentially life-threatening side effects under anti-amyloid-beta therapy. These are known under the term ARIA (amyloid-related imaging abnormalities), referring to edema or microbleedings in the brain (Filippi M et al, (2022)).
APOE genotyping prior to the start of the anti-amyloid (beta) therapy in early Alzheimer’s disease has therefore become crucial to assess a patient’s risk for side effects.
Our IVDR-compliant PCR-based EURORealTime APOE reliably detects the APOE ε2, ε3 and ε4 alleles in one reaction from genomic DNA isolated from EDTA blood samples.
Also keep in mind that – additionally to the EURORealTime APOE – we offer a convenient package to support Alzheimer’s diagnostics: ELISA and ChLIA products for the detection of the biomarkers amyloid beta, total tau and pTau to support the early recognition of the disease.
Learn more on our website: APOE | EUROIMMUN Alzheimer’s disease | EUROIMMUN

